The Breakthrough: ICHORtec’s Quantum FMB® Platform
At ICHORtec, we have introduced a new dimension to molecular precision — Quantum Fluorimetry of Molecular Binding (Quantum FMB®) — that transforms CRISPR design from a trial-and-error process into a predictive, physics-based science.
By directly measuring free energy (ΔG), the universal determinant of molecular binding, the Quantum FMB® platform identifies which guide RNAs (gRNAs) truly bind their DNA targets with optimal affinity and specificity — even down to single-nucleotide differences.
This capability closes the long-standing gap in CRISPR workflows between computational prediction and biological validation.
ΔG-Driven Precision Workflow
Our Three-Stage ΔG-Driven Precision Workflow transforms molecular discovery into a fully predictive, data-driven process — from computational design to preclinical validation. By directly measuring the free energy of nucleic-acid hybridization (ΔG), the platform ensures unmatched precision across the entire molecular value chain.
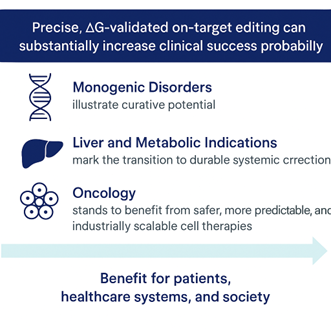
Quantum-Validated CRISPR: Redefining Therapeutic Potential
With Quantum FMB®, CRISPR transitions from statistical probability to energetic certainty.
In oncology, ΔG-validated editing enables safer and more effective immune cell reprogramming..
And across all therapeutic fields, it minimizes off-target risks, accelerates IND submissions, and reduces attrition throughout development.
Regulatory Pathway and Next Steps
 Quantum FMB® technology and the ICHORscope analytical system are progressing toward ISO 13485 and FDA RUO/IVD validation.
Quantum FMB® technology and the ICHORscope analytical system are progressing toward ISO 13485 and FDA RUO/IVD validation.
Current collaborations in Europe and the U.S. are establishing reference datasets for preclinical and translational applications in CRISPR verification.
During this phase, all Quantum FMB® systems and gRNA Nomination Services are available as Research Use Only (RUO) products under the ICHORtec Scientific Framework, ensuring data integrity, traceability, and reproducibility.
From Energy to Ethics: The Molecular Responsibility
At ICHORtec, we view the ability to measure molecular free energy not merely as a technological milestone, but as an ethical obligation.
When precision becomes measurable, inaction becomes indefensible.
Our mission is to make energetic truth — ΔG itself — the foundation of tomorrow’s medicine, ensuring that every therapeutic decision is based on measurable, reproducible molecular certainty.